We have here an interaction that might seem surprinsing: how can climate change act on the “ozone layer” ? Let’s recall first that climate change and “hole in the ozone layer” are two processes with distinct origins.
Let’s recall also that the ozone we are interested into here is the stratospheric ozone, that is the “good” ozone, the one that we will find in the stratosphere, between and 30 km above our heads. The “ozone layer” isn’t 20 km thick, of course, and actually it is not a layer – it does not ressemble a layer of snow on the ground – but just a concentration in ozone in the air which is higher at these altitudes than elsewhere in the atmosphere (see figure below).
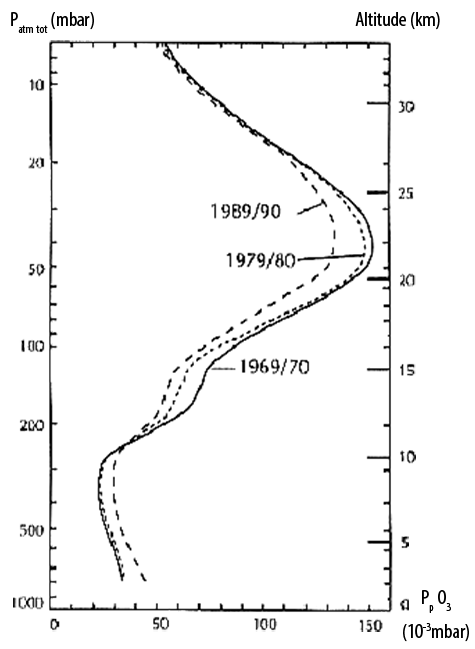
Partial pressure of ozone in thousandths of millibar (axe horizontal), plotted against altitude (vertical axis on the right).
The total atmospheric pressure is represented on the left vertical axis, in millibars. The three curves represent the ozone concentrations for three different years.
One easily sees that the concentration of ozone in the air never exceeds 0,5% : this value is a little low for a real layer !
One can also see that stratospheric ozone (the ozone in high altitudes) decreases with time, but that near the ground it is the opposite evolution that happens.
Source : Gérard LAMBERT, L’air de notre Temps, Seuil 1995
If we come back to the possible action of climate change on the depletion of stratospheric ozone, the chain of events works the following way:
- some human emissions (the CFCs) degrade stratospheric ozone (the one of the famous “ozone layer”) because of the chlorine that these coumpounds free in the high atmosphere. This process is permanently happening in the stratosphere.
- But this process can be particularly enhanced over the South Pole during austral spring, and then creates the famous “ozone hole”. Reasons are the following:
- When the south pole is plunged in the long winter night, the CFCs are not dissociated by the solar ultraviolets any more (see explanations on this other page on ozone)
- during the winter, temperatures in the stratosphere go down to a very low value (around -80°C), and this allows the apparition of clouds made of little crystals of ice (polar stratospheric clouds). Their ice is not pure, but also contains sulfuric and nitric acids.
- these crystals act as catalysts and allow the CFC to free their chlorine in various coumpounds that do not directly react with ozone. This process goes on during the whole austral winter, as long as these famous stratospheric clouds are present,
- during the austral winter, the air that stands over the south pole forms a king of giant vortex, with the consequence that the air in the vortex does not communicate any more with the air outside. Hence, the accumulation of chlorine remains confined over the south pole.
- When the sun reappears, its ultraviolets dissociate at once all the chlorine coupounds that accumulated during the winter, freeing atomic chlorine that instantly reacts with stratospheric ozone which can be depleted up to 90% in a couple of weeks.
- if the greenhouse effect increases, this will increase the temperature of the lower part of the atmopshere, but correlatively diminish the energy input in the stratosphere, that will globally cool down, and in particular will reach sooner very low temperatures during the austral winter.
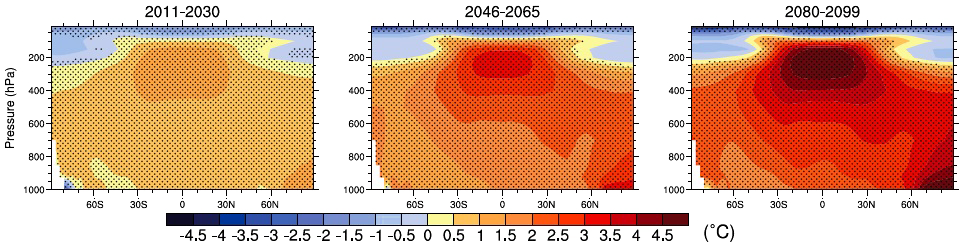
The above graph represents the evolution of temperatures with time in the free atmosphere, depending on the latitude and the altitude, for the A1B scenario (roughly a doubling of the emissions between 2000 and 2100).
The horizontal axis represents Latitude (Equator in the middle, otherwise graduated in degrees of latitude) and the vertical axis the pressure, graduated in hectoPascals (pressure decreases with altitude). The stratosphere begins at 200 hectopascals and at 0 we are out of the atmosphere !
Red is hotter than today, and blue to violet cooler. It is easy to see that the stratosphere (higher than 200 hectopascals) will cool everywhere, and particularly over the poles (90N and 90S).
Source : IPCC, 4th Assessment report, 2007
- This can lead to an increase of the time during which chlorine accumulation will be possible over the south pole, and as a consequence an increased depletion of ozone when austral spring comes.
- and most of all, such a depletion process could take place over the north pole, where the temperature of the stratosphere now begins to be low enough to allow the apparition of stratospheric polar clouds, that constitute an indispensable catalyst. If such a “hole” happened over the North Pole, it could be annoying for men and animals, because the austral mid-latitudes are not densely populated (the only emerged lands are New Zealand and the south of Argentina), while the mid-latitudes of the northern hemisphere harbor more than a billion people.
But, since it is now forbidden to manufacture CFCs, is there any more of them in the stratosphere ? Alas, yes: CFCs have a long lifetime in the atmosphere (decades to centuries) and the CFC concentration in the atmosphere is still close to its historical maximum. Furthermore there is a little chlorine in the stratosphere resulting from natural processes.
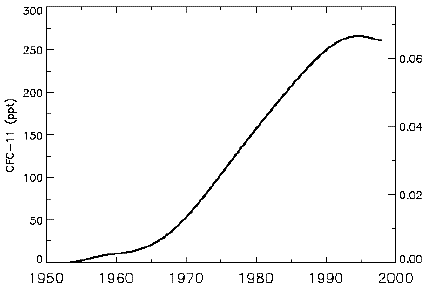
CFC 11 concentration in the troposphere (the troposphere is the lowest layer of the atmosphere, the one that is in contact with the ground and ends with the beginning of the stratosphere).
CFC 11 is the second most abundant CFC in the air. We are still close to the historial maximum in the troposphere, and, as a consequence of the delay that separates the maximum in the troposphere from the maximum in the stratosphere (it takes a while for these heavy molecules to spread from the troposhere to the stratosphere), the stratospheric concentration keeps rising.
Source : IPCC, 2001
With a kind of “chain reaction”, we can see that climate change might cause an additionnal ozone depletion in the stratosphere. In the extreme case of total vanishing of the ozone layer – fortunately impossible for the visible future – all superior life on emerged land would probably vanish.