I would not have supposed it that frequent when I started to investigate our “climatic future”, but there is a very frequent confusion that is made between two important environment problems that happen “above our heads”: the enhancement of the greenhouse effect and the “hole in the ozone layer”. This confusion even happens for some high-ranking members of the government or known journalists (I’ve got names !).
These two phenomena have certainly characteristics in common, that explain this confusion. Here are – among others – some of them:
- climate change and ozone destruction in the high atmosphere are two phenomena that derive from human induced emissions of particular gases: greenhouse gases in the first case, CFC in the second, the latter, in order to increase the confusion, being also a greenhouse gas !
- in both cases it refers to a global atmospheric process, meaning that the place where the gases are emitted is of no importance for the consequences, because the residence time of the gases in the air is long enough (decades or centuries in both cases) for the atmosphere to be well mixed shortly after the emissions happened (a matter of weeks to months),
- another consequence of the long residence time of the involved gases is that the processes are not reversible on the short term, if reversible at all – for climate change – before the disparition of men,
- in both cases an international agreement has been set up to try to solve the problem: the Montréal protocol in the case of stratospheric ozone (but this protocol says nothing about climate change) and the Kyoto protocol for climate change (with no additional decision concerning the restriction of CFC production and use),
- and at last the decreasing of high altitude ozone has an effect on the climate, even though it is not the major source of climate change: if the “ozone layer” gets thinner, less ultraviolets are intercepted in the high atmosphere, and therefore a little more solar energy reaches the ground, what intensifies the heating of the atmosphere from below, and therefore modifies the climate.
Differences between climate change and decreasing of the stratospheric ozone
But there are, of course, major differences between these two phenomena that are climate change and the “hole in the ozone layer”:
- Except for the CFC, the greenhouse gases, that cause the climate to change, do not perturb the ozone layer,
- The role played by the greenhouse gases is purely physical: they influence the energy exchanges in the atmosphere, but they do not generate a chemical reaction that would be the cause of the problem.
- On the opposite, it’s the “chemical role” played by the CFC that causes the problem for the high altitude ozone,
- The mitigation possibilities are not the same in both cases: for the ozone layer, the emissions involved (CFC) result from a restricted number of industrial activities (see below), what makes it pretty easy to fight against the problem, when for climate change it’s the use of energy, that is what fuels every little bit of our modern world, which is involved.
- At last, we are not at risk the same way with these two processes :
- in the case of the decrease of the ozone layer, the primary consequence is an increase of the amount of ultraviolet radiation that reaches the ground. This can cause direct sanitary effects (cancers…), but can also damage the ecosystems, particularly through an inhibition of the photosynthesis (but in order to experience serious problems it would be necessary to have a much larger decrease of the stratospheric ozone than today),
- a brutally enhanced greenhouse effect will primarily impact ground temperatures and the water cycle, with specific – and potentially severe – risks.
Differences between the ozone of the cities and the ozone of the layer
In order to complicate things a little bit more, there are two places where it’s possible to find ozone concentrations in the atmosphere:
- in the high atmosphere (in the stratosphere, to be precise), it’s the “good” ozone, the one that stops the energetic ultraviolets,
- close to the ground, it’s the “bad” ozone, the one that is responsible for the pollution of the cities in summer, and that burns the eyes and irritates the throats.
Do we speak of the same ozone ? Yes: it is definitely the same chemical coumpound ! Only in the high atmosphere we don’t breathe it, because we don’t live there, and we just see its positive effect. In the lower atmosphere it does intercept ultraviolet too, but there we breathe it !
It is also essential to indicate that the ozone near the ground and the ozone “far up” don’t mingle together. They result from two distinct cycles, have two distinct ways of appering and disappearing, and do not communicate one with another: an ozone molecule formed in the lower atmosphere will never move to the higher atmosphere, and vice versa.
- In the high atmosphere, the apparition and the removal processes are principally the result of ultraviolet radiation acting on oxygen molecules to create ozone, and then on ozone molecules to give back oxygen. Various coumpounds (including CFCs, precisely) can displace the equilibrium ozone concentration in a way or another (displace it towards a lower ozone concentration in the case of CFCs).
- In the lower atmosphere (near the ground), the apparition of ozone needs “precursors”, that are often pollutants (hydrocarbons, nitrous oxydes), that react together, under the influence of the sun rays (some radiative energy is therefore also required here), to lead to ozone. The removal of this ozone is purely chemical (ozone is a more potent oxydizer than oxygen, and quickly finds “something” to oxydize, then decaying to simple oxygen or a regular oxyde).
But while the stratospheric ozone decreases, the ozone near the ground increases everywhere !
Everybody knows, now, that stratospheric ozone is decreasing. But it is of common belief to think that the ozone near the ground increases only in cities in the summer. There is difinitively such an increase, but actually ozone near the ground has also increased everywhere and all the time.
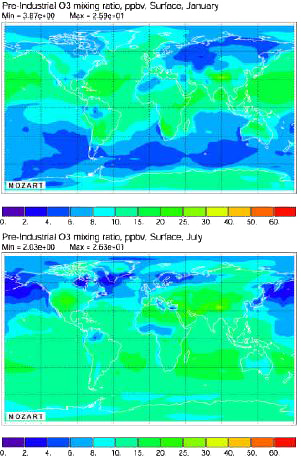
Average ozone concentration near the ground for the pre-industrial era (that is 1750), computed with the Mozart Model. Above, in january, below, in july.
Ozone concentrations are in parts per billion in volume, noted ppbv (1 ppbv = 1 m3 per km3 = 0,0000001%).
Source: Didier Hauglustaine, AFITE/LSCE lecture given in october 2003 at the Laboratoire des Sciences du Climat et de l’Environnement
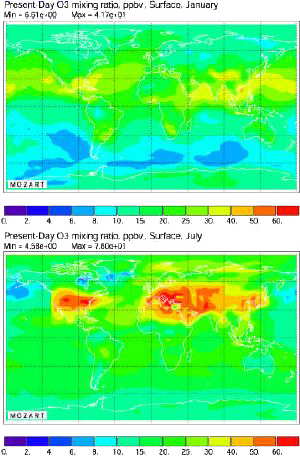
Same computation, but for present times (of course this calculation fits the data obtained from measurements where available).
The increase over the whole surface of the planet is striking: even over the middle of the Pacific Ocean has the ozone concentration increased.
Source: Didier Hauglustaine, AFITE/LSCE lecture given in october 2003 at the Laboratoire des Sciences du Climat et de l’Environnement
Is this increase anyhow linked to climate change ? Yes, in two ways:
- As ozone is a greenhouse gas, an increase of ozone near the ground will increase the greenhouse effect, and therefore will contribute to an increased climate change. In first approximation, ozone concentrations grows with the consumption of fossil fuel and the amount of forest fires.
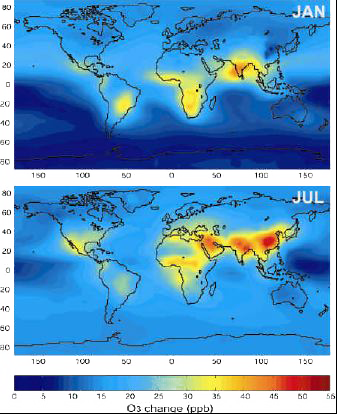
Possible increase of ozone near the ground, in ppbv, between 2000 and 2100, by combining the results of 10 different models.
The increase is particularly acute over China and India (a result of the projected increase of fossil fuel consumption in the scenario used), and over Africa (increase in forest fires).
For some regions, this increase will result in a doubling or a tripling of the present values.
Source: Didier Hauglustaine, lecture given in october 2003 at the Laboratoire des Sciences du Climat et de l’Environnement
- If we go a little upwards, but without getting into the stratosphere, ozone also increases, especially if we look at the consequences of air travel….
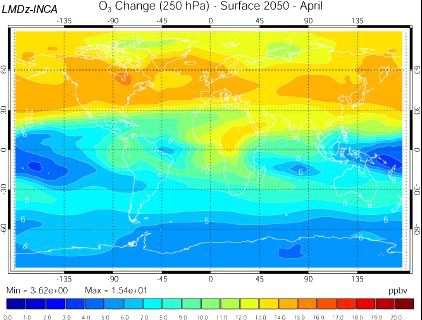
Increase of the ozone concentration 10 km above the ground in 2050, if the emission scenario follows the A2 path (which is a very pessimistic one, see emission scenarios), computed with the INCA model.
US are on the left of the map, Europe in the center, and Asia on the right.
Source: Didier Hauglustaine, lecture given in october 2003 at the Laboratoire des Sciences du Climat et de l’Environnement
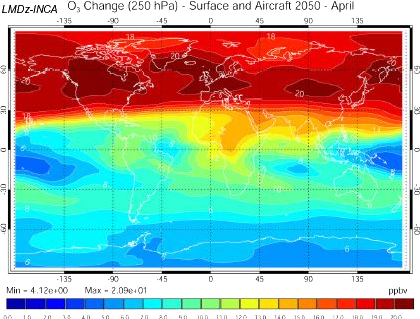
Same computation, but including the emissions from planes, that follow the Fa1 emission scenario of the IPCC 1999 special report on planes.
Source: Didier Hauglustaine, lecture given in october 2003 at the Laboratoire des Sciences du Climat et de l’Environnement
- If we get back near the ground, ozone is a known inhibitor of the growth of plants. An increase of ozone concentrations near the ground could (will ?) therefore weaken the photosynthesis of plants, a natural process that removes CO2 from the atmosphere. This effect is not taken into account in the simulations that lead to the “famous” 1.4 to 5.8 °C increase of the annual mean temperature in 2100. Taking it into effect might lead to an accelaration of climate change, just as what is feared with the carbon cycle, but of an unknown magnitude.
The gases involved in the “diminishing of the ozone layer”: the CFCs
The abrevation CFC means “chlorofluorocarbon”. This familly of gases comprises numerous representatives, that are all made the same way: to obtain a molecule of chlorofluorocarbon, one must start from a molecule of hydrocarbon (like methane or propane or butane, that are the most common cooking gases, or octane, that can be found in petrol for cars, or benzene…) and substitute all the hydrogen with a combination of chlorine (explaining the “chloro” radical in the word chlorofluorocarbon) and fluorine (explaining the “fluoro” radical in the word chlorofluorocarbon).
The general formula of a molecule of CFC is therefore CxClyFz: it contains only carbon, chlorine, and fluorine.
CFCs are simultaneously potent greenhouse gases (they efficiently intercept infrared radiations emitted by the surface of the earth, and have a lifetime of several hundred years in the atmosphere), and precursors of some coumpounds that destroy the stratospheric ozone. It’s only the second effect that has justified the progressive ban of their production (and use, of course !).
Why were they used, then ? Of course, at the time of the rise of their production, their effect on stratospheric ozone was not known. Their success comes from the fact that they constitute very stable molecules (much more than hydrocarbons), which means that they have sanitary or security advantages for human beings. On the opposite, what we designate under the word “pollutant” is classically a very active chemical coumpound, susceptible to react with a great number of another coumpounds, and therefore to generate non wished effects among living being that inhalate it, or eat it, or touch it.
A coumpound that reacts with nothing, or almost nothing, if we ever breathe or eat some, chances are that we won’t even notice it. CFCs have therefore replaced, for various industrial applications, gases that had sanitary or security inconvenients, that is were potentially toxic or dangerous:
- they replaced ammonia as frigorific fluid (that is for filling fridges, air cons and freezers),
- they were used as expansing gases to make plastic foams (for example expansed polystyrene),
- they were used as solvants in the semi-conductor industry,
- as they are not flammable, they served as propeller gas for sprays, or to fill fire extinguishers,
- etc…
But it’s preciseley because these molecules are “solid” that they generated the environment problem known as the “hole in the ozone layer”: as they are not reacting with anything once in the atmosphere, they remain there long enough to have the time to spread to the stratosphere (what takes a long time because they are generally heavy molecules), where they are broken down by ultraviolets sent by the sun, thus freeing the chlorine they include, which then starts a complex set of chemical reactions leading to the destruction of high altitude ozone.
This ozone destruction happens in a very massive and spectacular way over the South Pole when the spring begins in the southern hemisphere: almost all the stratospheric ozone present over the South Pole then disappears in a couple weeks.
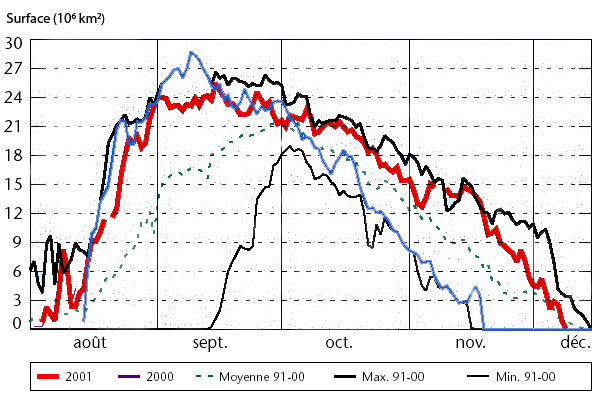
Day to day evolution of the surface of the “hole in the ozone layer” over the South Pole, in millions of km2, for various years since 1990. As a comparison basis, one can remember that Northern America’ superficy is worth 25 million km².
The “hole” is defined as the surface for which the ozone concentration is at most half of its usual value (for those who like details, the normal value of the ozone concentration is roughly 400 Dobson units, and the “hole” designates the region where the value is less than 220 Dobson units at most). And at last, for those who did not guess by themselves, “août” means august, and “moyenne” means average.
It is easy to see that the extension of the hole for the recent years (2000 in blue ; 2001 in red) is over the average for the years 91-00 and close to the maximum for that period. The problem is not behind us !
From World Meteorological Organization, report N° 940, quoting the Climate Prediction Data Center of the NOAA, 2002