As everybody knows (or should know !), man is now putting significant amounts of CO2 into the atmosphere, though our emissions are taking place into a natural carbon cycle much more complex, which includes exchanges between the atmosphere and the ocean.
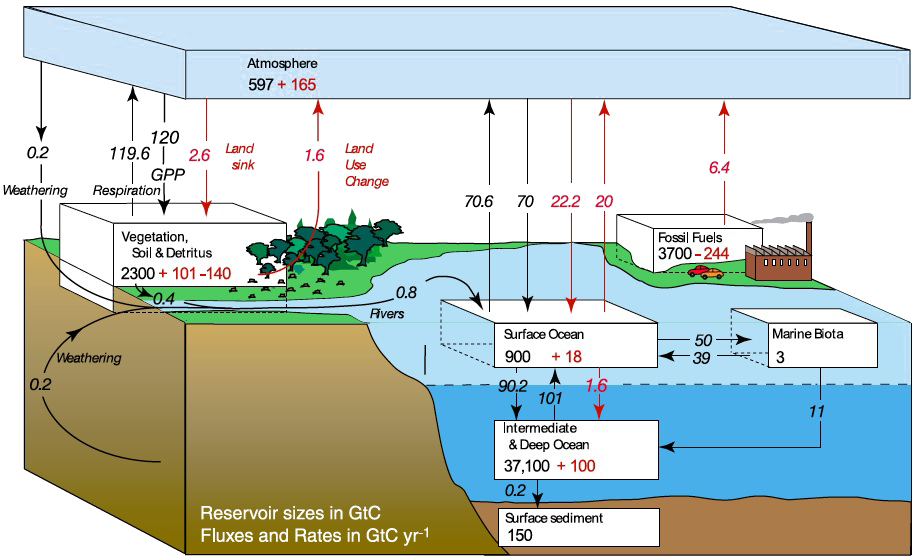
Annual flows and existing stocks of carbon, in gigatonnes (billion of tonnes) of carbon.
Source : IPCC, 2007
The CO2 exchanges between the atmosphere and the surface ocean (roughly 90 billion tonnes of carbon each way per year on the above graph) do not owe much to the existence of a marine life : if we suppressed all fish and all whales, it would definitely generate some inconvenients, but not a slowdown of the CO2 exchanges between the atmosphere and the surface ocean ! These exchanges are essentially a consequence of the existence of large scale marine currents, that cool down water masses in some places (like the water of the Gulf Stream that shifts towards the North Pole), and heats up the surface ocean in some other places (for example the water of the Labrador Current that shifts away from the North Pole).
Indeed, CO2 dissolves better in cold water than in warm water (if one wonders why, the best answer will probably be “because that’s how it is” !), so that where water is cooling, some CO2 goes from the atmosphere to the ocean, when where water is heating up, some CO2 goes from water to the air. This explains why, in a globally warming climate, the oceanic sink will have a tendancy to weaken : the absorption of CO2 will be lower with cooling water masses remaining a little warmer than before (on average), while the emissions of the warming water, that will reach temperatures a little higher, will be more important.
But once absorbed by seawater, this CO2 will not remain as such for the most part, no more than the CO2 “absorbed” by the continental ecsystems remains under the form of CO2. Once dissolved in seawater, part of the CO2 reats with water to form hydrogenocarbonate ions (once named bicarbonate ions), HCO3-, then carbonate ions, CO3–.
The successive reactions are thus as follows :
CO2 + H2O → H2CO3 → HCO3- + H+ → CO3– + 2H+
Actually, as any chemical reaction, these can happen either way (it depends on the initial conditions, and for given initial conditions there is of course a way which is privileged). One should then rather write :
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+ ↔ CO3– + 2H+
Well, old memeories from the chemistry class might allow the reader to remember that a coumpound that “produces” H+ ions is named… an acid. What the above reaction means is that the dissolution of CO2 in sea water makes this water more acid. This property explains why, in old times, the CO2 was called “carbonic acid” (it is for example the expression used by Arrhenius in his premonitory article on how the industrial era would bring a global climate change).
What the chemical reaction above doesn’t say, however, is that if we have more CO2 in the air, we will get more in the ocean. We are not facing chemistry here, but thermodynamics (ouch ! here are more and more barbarian expressions !). In other words, if we have more CO2 in the air, it “penetrates” in larger quantities in the underlying water.
From all that preceedes, one can thus deduct that if we increase the atmospheric CO2 concentration, what we are definitely doing right now, not only will we change the climate, but we will also make the ocean more acid.
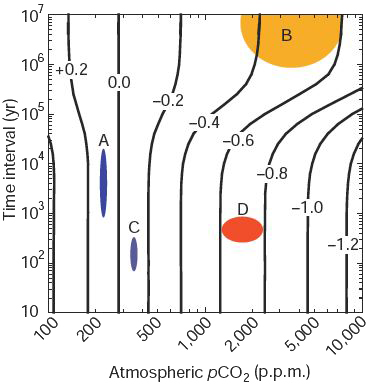
Intercomparison of the past pH changes of the surface ocean (same colour code than for the graph on the left). The “vertical” curves give the surface ocean pH loss depending on the rate of atmospheric CO2 increase. For example, an increase to 2000 ppm of the CO2 concentration in a couple million years generates a decrease of the pH limited to 0,5 unit, but if it happens in a couple centuries, the ocean will become more acid, losing 0,8 pH unit.
A – variation of the acidity of the surface ocean between glacial ages and interglacial ages (such as today) : pH±0,1 in several thousand years
B – variation of the acidity of the surface ocean during the last 300 million years : pH ±0,5
C – variation of the acidity of the surface ocean during the last century, as a result of the CO2 emissions already done : pH±0,1
D – possible variation of the pH as a result of future emissions, in the “high” case : with 1000 to 2000 ppm extra of CO2 in a couple of centuries, the ocean would loose 0,5 pH units or more. Such an evolution would be without precedent for million (tens of million ? Hundreds of million ?) years, because the rate of increase of the CO2 in the air would be unprecedented. Such an evolution – in several centuries – is not impossible if the continental CO2 sinks are turned into a source.
Source Caldeira & Wickett, Nature, 2003
One can see on the aabove graph that with a CO2 concentration in the air that would reach 1000 ppm, which is definitely in the high end of the bracket of emission scenarios, the ocean acidity could increase by more than 0,5 pH unit (more exactely the pH would decrease by 0,5 unit).
Even with emissions considered as “moderate” (leading to an atmospheric CO2 concentration reaching 550 parts per million in 2100), the ocean would loose 0,2 to 0,3 pH units in 2100 compared to now.
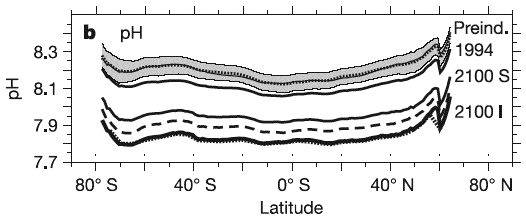
pH of the surface ocean (vertical axis) depending on the latitude (horizontal axis ; 90°S = South Pole, and 90°N = North Pole) and the atmospheric concentration of CO2.
The grey zone at the top – with a dotted line in the middle – gives the range of pH for the pre-industrial period (1750), simulated with models. The thick black line with the year 1994 wrtitten on the right gives the pH measured in 1994, and the bottom lines give the pH in 2100 depending on the emission scenario (I stands for Is92a, 800 parts per million of CO2 in 2100, and S for S650, with 550 parts per million in 2100 then 650 when stabilization occurs).
Source Orr et al., Nature, september 2005
This would have significant consequences on the marine life, even with no associated climate change. As usual, some consequences can already be “theoretically” identified, and for others it will be a surprise, because nobody will have thought of them before they happen (which is normal when a situation is new).
Among the marine organisms that will feel a change of the acidity of the surface ocean, one will find corals, because these animals build up a skeletton made of lime, and the chemical reaction leading to the formation of calcite becomes harder to perform when the water gets more acid. May we get back to some simple chemical equations ? Then here is what normally happens when the coral builds up its skeletton :
Ca++ + CO3– → CaCO3
In other terms, the coral is using calcium ions dissolved in seawater and combines them with carbonate ions to “produce” calcium carbonate, which is nothing else than the “scientific” name of …. lime.
We have seen above that the CO2 that dissolves into seawater generates some “descendants” that are hydrogenocarbonate (HCO3-) and carbonate (CO3–) ions, and that the respective proportions of each compound at equilibirum depends on the conditions at the time. In the tropics (where corals live), hydrogenocarbonate ions represent 85% of the dissolved carbon, and carbonate ions 15%. If the acidity increases, the respective proportions will change, and more precisely the share of the carbonates decreases (graph below).
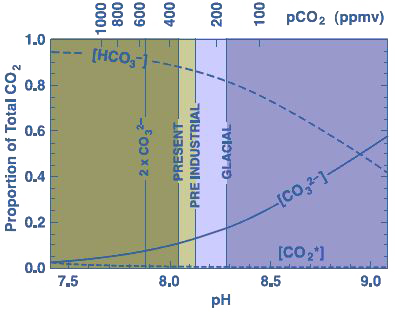
Respective proportions of carbonate ions, hydrogenocarbonate ions, and dissolved CO2 depending on the acidity of seawater. The present pH of seawater is around 8 (seawater is therefore slightly alcaline).
If the CO2 increases in the air, the proportion of carbonate ions in the water decreases, and the formation of calcium carbonate is more difficult.
Source : Coral reefs & Global climate change, Pew Center on climate change, 2004
Well it is only carbonates that are used by marine organisms that need to build up a shell or a limestone skeletton, and we can quite reasonnably consider that if carbonates are less available in seawater, all the concerned life will have a harder time. Indeed, laboratory experiments show that with a doubling of the CO2 compared to today, calcification (the “production” of limestone by living organisms that do so) slows down by 10 to 40%, and this applies both to corals and to the phytoplankton algae that have a shell.
Simulations done with coupled models (climate and carbon cycle) show that the concentration in carbonates could be divided by two at high latitudes (from 100 to 50 micromoles per liter of seawater) by 2100.
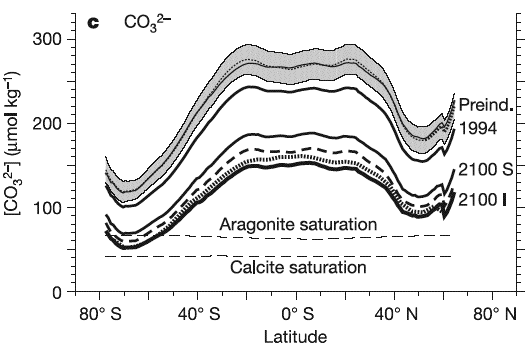
Carbonate (CO3–) concentration depending on the latitude, for various times. The thick black line with the year 1994 wrtitten on the right gives the concentration profile measured in 1994. The grey zone at the top – with a dotted line in the middle – gives the range of values for the preindustrial times (1750), and the lines with the letters S and I give the simulated carbonate concentration for 2100 depending on the emission scenario (I stands for Is92a, 800 parts per million of CO2 in 2100, and S for S650, with 550 parts per million in 2100 then 650 when stabilization occurs).
The hashed lines at the bottom with “aragonite saturation” and “calcite saturation” give the minimal values of carbonate concentration that are necessary for the synthesis of aragonite and calcite (by microorganisms) to happen. Aragonite and calcite are two varieties of lime.
We can see that even with a scenario “not too high”, life will become impossible at high latitudes for micro-organisms that need to ceate a shell or skeletton from aragonite.
Source Orr et al., Nature, September 2005
In all cases, the pH change which is expected is very brutal compared to the historical values over millions of years.

Past values for the oceanic pH over the last 25 million years, and possible values for the future.
Source Pearson et al., Atmospheric carbon dioxide concentrations over the past 60 million years. Nature, 2000, cité in Turley et al. DEFRA 2006
What the above graphs suggests is that if the ocean pH decrases by more than 0,8 units (turning the sea almost acid), we would make all life difficult to all marine organisms that needs to produce lime, and that includes, apart from corals, all molluscs (oysters, mussels, etc), all crustaceans, a large fraction of the phytoplankton…. Hopefully, we are not there for the moment, and given the amount of CO2 in the air it requires to obtain such a drop of the pH it is not for tomorrow, but for after-tomorrow the risk is more than serious….