NB: all pictures come from the article “Du kérogène au pétrole et au charbon: les voies et les mécanismes des transformations des matières organiques sédimentaires au cours de l’enfouissement, Bernard Durand, Mem. Soc. Geol. France, N.S., 1987, pp 77-95. I warmly thank the author of this article for his kind authorization to reproduce them.
“Normal”, or, in the oil business langage, “conventional” oil, designates a liquid mainly composed of molecules of hydrocarbons, that is made only of carbon and hydrogen, with anything between a couple and a couple hundred atoms of carbon per molecule. This “normal” oil also contains, in variable proportions (15 % on average), more complex and heavier molecules including oxygen, nitrogen and sulfur, called resins.
All oil has been formed from living organisms (algae, plankton, sometimes continental vegetation…) that lived a very long time ago. Each reservoir in the world yields an oil that has its own characteristics: just as there are no two totally identical human beings on earth, there are not two oilfields that contain exactely the same liquid.
The making of oil is the result of a long process that requires a succession of particular stages:
- Organisms are dying all the time on the surface of our planet. These organisms (including us !) are mainly composed of carbon, hydrogen, nitrogen and oxygen. One of the characteristics of life is the ability to sustain complex molecules (proteins for example), and when death occurs the delicate combinations are “broken” – the proper word is decay – and almost all sub-components are recycled and reused quickly by the biosphere. It is the case, for example, of the carbon dioxide generated by this decay that is used by living plants, or nitrogen compounds that enrich the soil, etc.
- However a small fraction of the total (less than 1% of the dying biomass) sediments, which means that it gets included in the forming rocks or mineral layers of sedimental origin. The sédimentation process is permanent at the botton of the oceans (particularly for coastal areas) and lakes. It definitely produces little effects over a man’s lifetime, but is of crucial importance over “geolical” time scales (a couple million to a couple billion years).
- All the sediments formed, though they might seem totally mineral, therefore include a more or less important fraction of organic matter (around 1% on average), that becomes “trapped” in the forming layer. This organic fraction undergoes a first transformation under the effect of bacterial activity at the beginning of the sedimentation process, which leads to the apparition of a solid compound named kerogen, disseminated – given its small proportion – under the form of small threads in the mineral part.. The latter is named “mother rock”
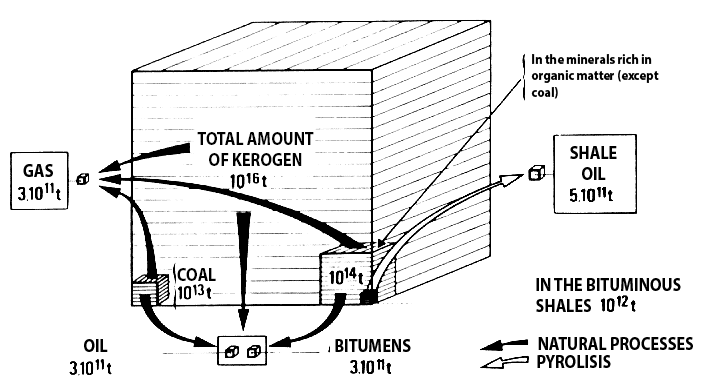
Total mass of kerogen on planetary scale
Though it is generally present in very small proportions in the sediments, all kerogen on earth represents a total mass of 10.000.000 billion tonnes. 0,1% only of this kerogen (that is a thousandth of the total organic matter present in sediments) will turn into coal (which still represents 10.000 billion tonnes !), and gas and oil each represent 0,003% of the total kerogen in rough figures (which still represents a couple hundred million tonnes).
- Because of tectonics, that is a very slow convective motion of the Earth crust, sediments eventually slowly “sink” into the deeper ground. The ambiant temperature then increases, because of geothermal heating (incidentally geothermal energy is a kind of nuclear energy: it results from the energy freed by the natural radioactivity of the inner earth !). The burrial rate being variable, the temperature of the sediment increases by 0,5 to 20°C per million years.
- When the ambiant temperature reaches 50 to 120 °C (the minimum temperature required depends on the age of the mother rock, it is complicated !), kerogen undergoes a decomposition of thermal origin, pyrolysis (provided there is no free oxygen around, otherwise the kerogen is just oxidized). In a first stage, the decomposition produced water and CO2 (in amounts that depend on the initial proportion of oxygen) that are expelled from the kerogen. As the temperature increases with time, the kerogen will expell liquid hydrocarbons (it is the famous oil) and “natural” gas (let’s note that oil is as much “natural” !). The deeper the sediment has gone, and the higher the fraction of gas produced, because the pyrolysis has lasted for a longer time before expulsion, or has decomposed partially the liquid hydrocarbons themselves. Given the slow burrial speed, a couple million years are required for the kerogen to be partly transformed, under the effect of heat, in oil, gas, CO2, and water.
- Each little kerogen thread of the mother rock will thereform transform into a mixture of water+liquid hydrocarbons+gas+solid residue with a high proportion of carbon (because most of the hydrogen has gone in the water, liquid hydrocarbons and gas). Only pretty watertight mother rock (hence with a fine grain) can hold the kerogen long enough for it to transform in a large proportion, but a consequence of this watertightness is that the hydrocarbons resulting from the pyrolisis first remain very scattered.
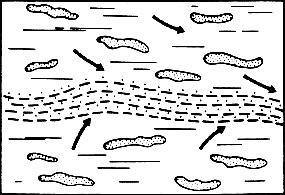
Stage 1 of kerogen transformation, at the begening of pyrolisis.
Each little thread of kerogen produced wter that is sometimes expelled under the pressure resulting from the masse of the upper layers above the sediment.
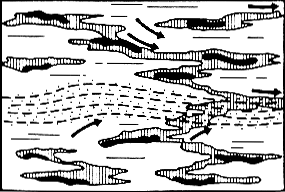
Stage 2 of kerogen transformation, during pyrolisis.
Each little thread of kerogen begins to produce hydrocarbons.
- The production of hydrocarbons is therefore a normal long term consequence of sedimentation as soon as there is an organic fraction in the initial mixture, but without a process allowing to “concentrate” the very diffuse gas and oil resulting from this decay, there would not be a single oilfield or gasfield in the world.
- It’s the apparition of gas, as the kerogen is brought to increasing temperatures (as a result of burrial), that will eventually cause the pyrolisis to stop. Indeed, the pressure of the gas in the little cavities that contained the initial kerogen increases with depth (because of rising temperatures), and when the pressure becomes sufficient to overcome the “airtightness” of the mother rock, the liquid and gaseous fractions are progessively expelled from the mother rock. This expulsion is called the “primary migration” by the oil geologists. When this “primary migration” happens, the mother rock can be 1 million to 1 billion years old, the most frequent age being around 100 million years: oil is therefore a renewable energy… if we can wait several ten million years before burning it !
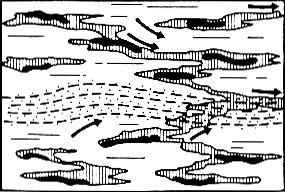
Stage 3 of kerogen transformation
Each little thread of kerogen has produced about all the hydrocarbons it could (there is almost no hydrogen left in the sediment). Under the effect of gas pressure, the “primary migration” begins.
- After they have been expelled from the mother rock, hydrocarbons (and water) begin what is called a “secondary migration”: they “ooze” along the permeable layers that are located next to the mother rock (which is pretty watertight, as explained above), and move towards the ground under the effect of the pressure caused by the layers located above.
- If nothing stops this upward movement, hydrocarbons eventually get to the ground (or just under for liquid hydrocarbons), where they are decomposed by bacteria and lead to the formation of bitumens. Bituminous sands of the Athabasca province, in Canada, that constitute the largest known accumulation of bitumens in the world, are a good representative of this stage of the evolution of “oil”. In a way, we are facing there a compound “older than oil”. These “leaks” to the surface are very frequent, and as they may come either from mother rock, or from reservoirs that loose some oil (see below), they have been widely used as possible signs of oilfields when the history of oil began. These leaks from reservoirs sometimes bear the name of “dismigration”.
- In order to get a minable oil deposit, it is necessary that the liquid hydrocarbons “concentrate” somewhere before they get to the surface, which practically requires that they be stopped during their migration by a “trap”. Such a trap is another watertight layer that generally forms some kind of “circumflex accent” above the porous layer when the oil circulates. It can be a layer of salt, of argile, etc. Because of their respective density, the water expelled from the mother rock accumulates below the oil, and gas above. At this stage, the reservoir si said to be of “conventional” oil. The rock that holds the oil is called a reservoir.
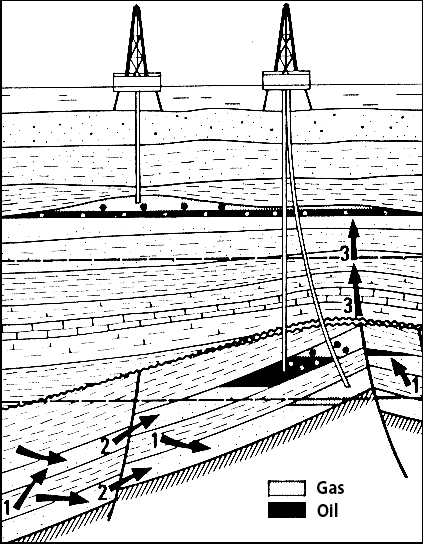
Overall picture of oil formation
1 – Primary migration
2 – Secondary migration, through pores or fractures
3 – Dismigration: oil “escapes” from a reservoir where it previously accumulated.
- When the kerogen has produced all the hydrocarbons it could produce, it means that is has lost all its initial hydrogen. There remains a compound close to coal, but not mineable because it is still disseminated in the mother rock where it represents less than 1% .
- But the history of our oil is not over ! Indeed, the oil reservoir is still taken in the tectonics, and therefore also inexorably sinks deeper and deeper, and gets heated more and more. As a result, the oil will undergo a second pyrolisis, which is a little the equivalent of a distillation in a refinery. This pyrolisis will produce gas and a particular variety of bitumen, in increasing amounts with time and temperature.
- If the reservoir is well tight, this new burrial will lead to the formation of a dry gas reservoir, that is a rock with only gas (this explains why in sedimentary basins the dry gas reservoirs are generally localted below the oil reservoirs). If the reservoir is not well tight, the gas escapes and only the bitumen (or asphaltes) remain in the porosities of the reservoir rock.
- Coal is a particular variety of kerogen, that forms from remains of superior plants (trees, ferns…). It is a kerogen that has the characteristic of being dominant in the sediment instead of being a very a small fraction of it. The first stage of the sedimentation process leads to peat. During the burrial, the pyrolisis then creates lignite, then coal, then anthracite, which is almost pure carbon, with almost no hydrogen (as it is the ultimate stage of the pyrolisis anthracite is generally the deepest of all coals). Just like other kerogens, coal produces oil and gas during its burrying in the underground, though in lesser quantities regarding oil. The methane issued from the process and which has remained adsorbed on the coal will be called… firedamp.

Overall picture of coal formation.
The right axis mentions time, in million years before present, and the left axis depth, in km (for each graph).
Each drawing represents a stage of coal formation over time, starting from the top (the upper drawing therefore represents the system that will yield coal 320 million years ago).
The organic matter (concentrated since it comes from piles of dead plants) goes through the following stages: peat – lignite matte – lignite bright – hard coal – anthracite. Each time the carbon content increases.
V1 and V2 represent a same initial organic matter that will produce Veins (hence the V) or seams of coal. The type of coal in the vein (A, H, etc) is mentionned in parenthesis. The successive drawings show how these veins evolve and move in the crust (because of tectonics) with time.
Source : B. Durand, Energie et environnement, les risques et les enjeux d’une crise annoncée. EDP Sciences, 2007
What liquid hydrocarbons are we able to extract ?
At the beginning of oil industry, the oil that we were able to extract was “conventional”, that is a liquid produced by the pyrolysis of kerogen, that then had the good idea to concentrate in a reservoir.
Getting this oil is pretty easy : it requires that a tube is forced into the reservoir, and then part of the oil spontaneously gets out under the pressure of the associated gas, and part of the rest can be “pumped” with various techniques that keep sophisticating. With this “conventional” oil, the extraction requires, on average, a couple % of the energy that is enclosed in the oil obtained.
But oil companies are more and more interested by “unconventional” oil that designates :
- bituminous sands and extra-heavy oil, that correspond to oil that has lost its volatile elements. It is therefore oil which is “older” than conventional oil, and that degraded close to the surface, as explained above, thus getting richer in heavy molecules,
- bituminous or tar shales that designate – wrongly because there is no tar in them – a mixture of rocks and kerogen that has not undergone a pyrolisis. It therefore corresponds to fuels that are “younger than oil” in the transformation process, and these resources should rather be part of the coal inventory, just like peat or lignite. These bituminous shales can yield synthetic oil by undergoing a pyrolisis (at 500 °C in order not to wait for a million years) in a plant, but the net balance regarding energy is poor (most of the time it is even negative, which means that the energetic content of the oil obtained is inferior to the energy spend to get it).
In all cases unconventional oil is something very thick (pasty), sometimes even solid in the conditions encountered in the reservoir, and often mixted up in minor proportions with rock. The deposits are much more difficult to exploit than for conventional oil:
- if it is extra-heavy oil, or bituminous sands, it is necessary to inject steam under pressure (to turn the oil fluid by heating it, and allow it to get out under the pressure of the steam), what requires to invest into the extraction a couple tens % of the energy that will be enclosed into the oil extracted,
- For bituminous shales, the fuel extraction is more like mining, and the fuel might represent only a couple % of the rocks that contains it. Some geologist recommend never to take these shales into account when calculating reserves.
It is also possible to extract gas that has been formed in a mother rock and never migrated afterwards: “shale gas“.